Because clinical testing is a time-consuming and costly process, we have designed the Datacapt tool to make your life easier. Datacapt, a platform created for and by experts in the field and specialized in cosmetics, Personal Care, Medical Devices, will enable you to optimize your work and save up to 8 hours a week.
Move up a gear in 4 steps
1. Replace your case report forms and paper surveys. Create your studies and forms in a few clicks (eCRF/ePRO)
Managing your studies and data on paper is a time-consuming and resource-intensive process. With Datacapt, creating your eCRFs and ePROs (surveys) has never been easier.
- Choose up to 16 question types and quickly build all your forms.
- Organize the forms and questions in any order you wish according to your protocol.
- Add repetitive measures, logical conditions and real-time data validation/consistency.
- Save and reuse your eCRF/ePRO forms in one click.
The Datacapt builder allows anyone to easily design and customize their own studies. You can start from scratch or use your templates in a few clicks and in record time.
Create your structure and questions according to your protocol.
The voluntary questionnaires/surveys (ePRO) are built in the same way as the eCRF forms. This allows us to harmonize and simplify the use of our platform.
“No more paper forms or complex solutions using specific coding, we save time and are 100% autonomous.”
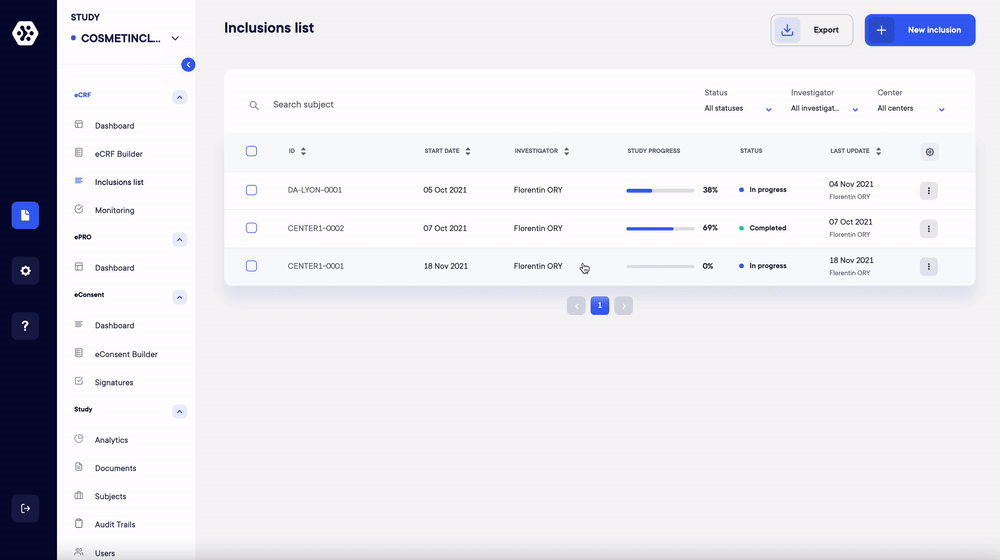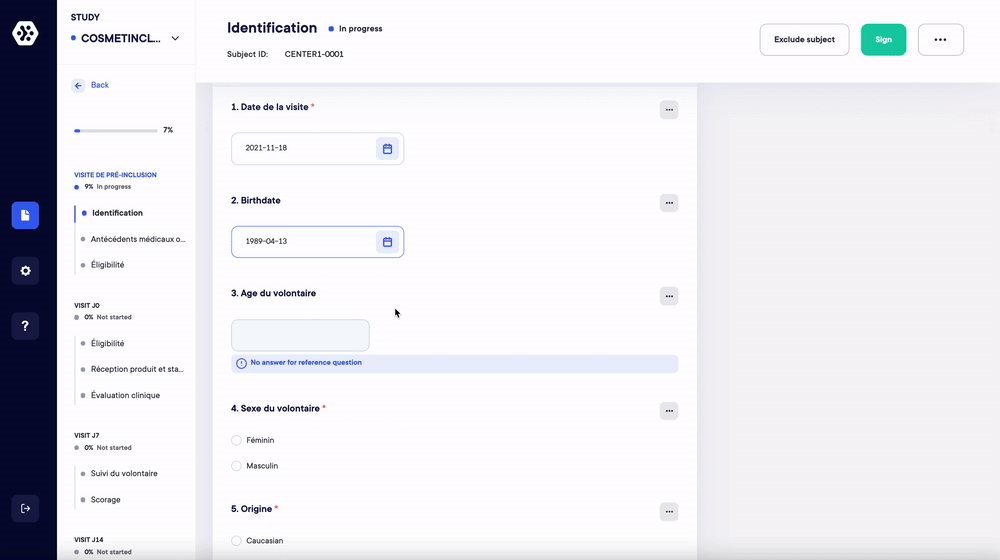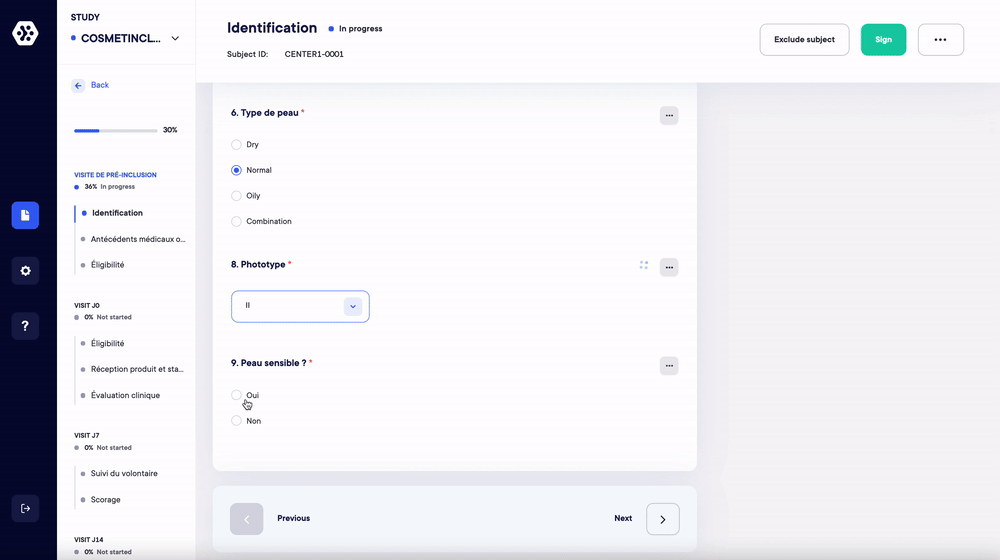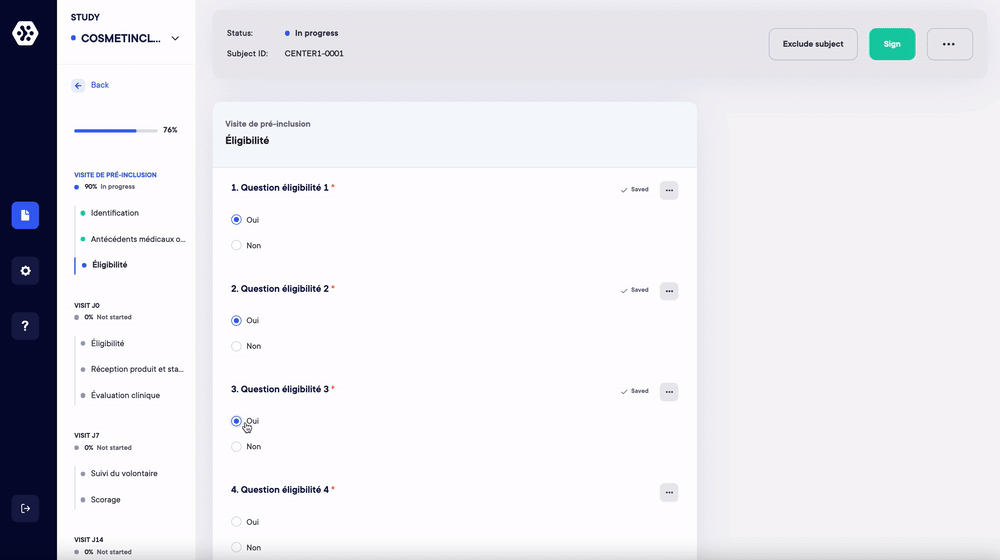
2. Collect your eCRF data and send surveys automatically
Once your forms are designed, collect all your data through a flexible and intuitive interface. Do away with the heavy logistics of paper forms! Our solution saves and controls all your data in real time to improve quality and speed.
Thanks to Datacapt, you can also send out your participant surveys and daily logs while monitoring data collection in real time. You also have the possibility to plan automatic reminders. Volunteers use their phone, tablet or computer to answer the questionnaires and this, increases their commitment.
Finally, for your multi-center studies, the platform allows individual investigators to collect and monitor form data in real time at multiple sites.
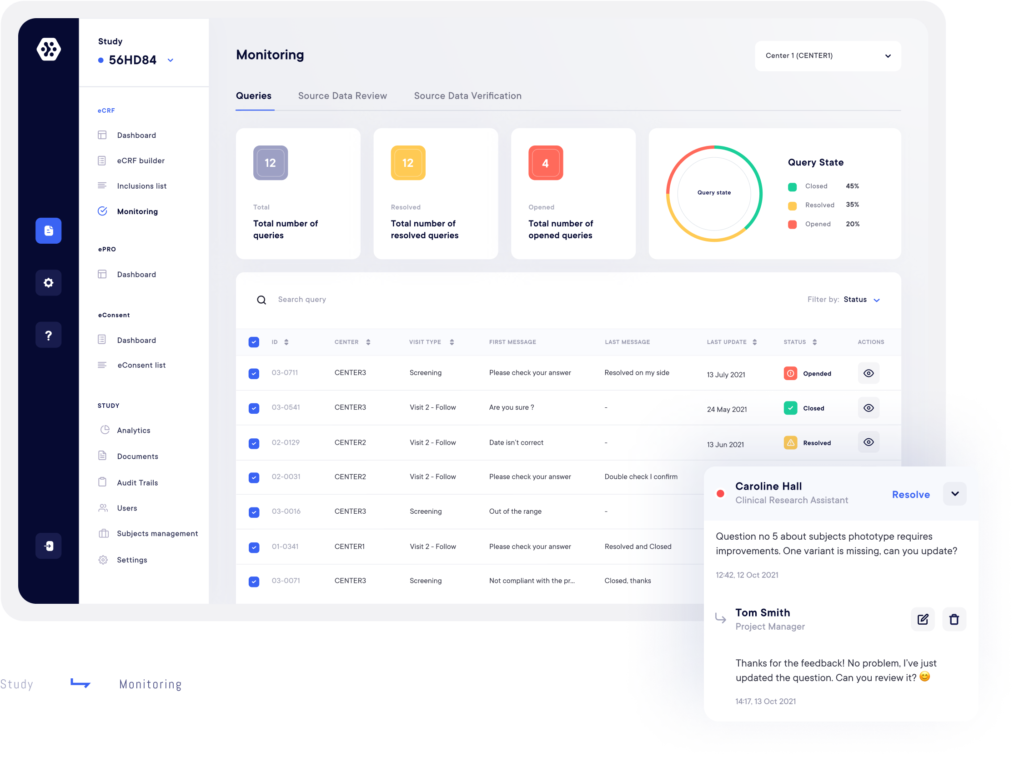
3. Monitor and track your data in real time
The Datacapt solution has many features that allow you to manage the collection of data in its entirety:
- Get an overview of the progress of your study and all your forms with interactive dashboards.
- Take advantage of automatic data validation and consitency checks and reduce human error.
- Electronically sign your forms: this allows you to do a partial freeze of the base and lock your data at any time.
- Access a monitoring page to view all queries for each volunteer.
- Benefit from centralized, complete and detailed data traceability.
4. Export and analyze your data with ease
No more double data entry or retyping data into multiple Excel spreadsheets. Access all data directly from our centralized platform..
Export your data in one click and in different formats according to your needs for a quick analysis. This will allow you to save a considerable amount of time, reduce the number of tasks to be managed and gain in quality while reducing human handling.
Datacapt, a complete and innovative platform
Datacapt also offers innovative modules for your clinical trials. Take advantage of eConsent (electronic consent) and eConsult (teleconsultation) and open up the field of possibilities for conducting your clinical tests.
The platform allows you to manage pre-inclusions, inclusions, follow-ups, visits, adverse events and much more.
Because choosing Datacapt is also choosing security!
During a clinical study, a lot of data is collected. This information must be highly secured, controlled and accessible only to authorized persons.
Certain regulations on security and confidentiality are unavoidable and must be applied by all actors: RGPD, CNIL, 21 CFR PART 11, ICH GCP… This is why using a digital solution such as Datacapt allows you to comply with all the regulations in force, unlike the use of paper or Excel spreadsheets, outdated methods that do not guarantee data security and inegrity.
With Datacapt, a platform fully adapted to your needs, you will have a powerful tool that will time-saving, improve the quality and traceability of your data and enable you to significantly reduce the cost of your clinical trials.
Discover Datacapt quickly and test it for free: https://www.datacapt.com/en/contact-demo/
CONTACT
Florentin Ory
Directeur associé
Tel. 06 77 38 02 61









 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.