One of the most common claims is “dermatologically tested”. Despite the familiarity you should have with this statement, are you sure to know exactly what it does mean?
According to The EU Guidelines to Commission Regulation (EU) No 655/2013, “it implies that the product has been tested under the supervision of a dermatologist”.
This does not clarify what kind of test it relies upon, but as the guidelines report shortly after “Depending on the presentation of the claim, it may refer to a specific efficacy or tolerance of the product.”
The use of the claim “dermatologically tested” for cosmetic products was also assessed by the European Court of Justice (Case C-99/01).
The Court clarified that “the average consumer’s expectation of such a claim is that the product underwent tests intended to study its effects on the skin and that the results of those tests were positive and showed that the product was well tolerated”.
That said, we should more likely say that the most suitable test will investigate mainly the safety of the product, even if the “dermatologically tested” claim may also embrace a test for effectiveness.
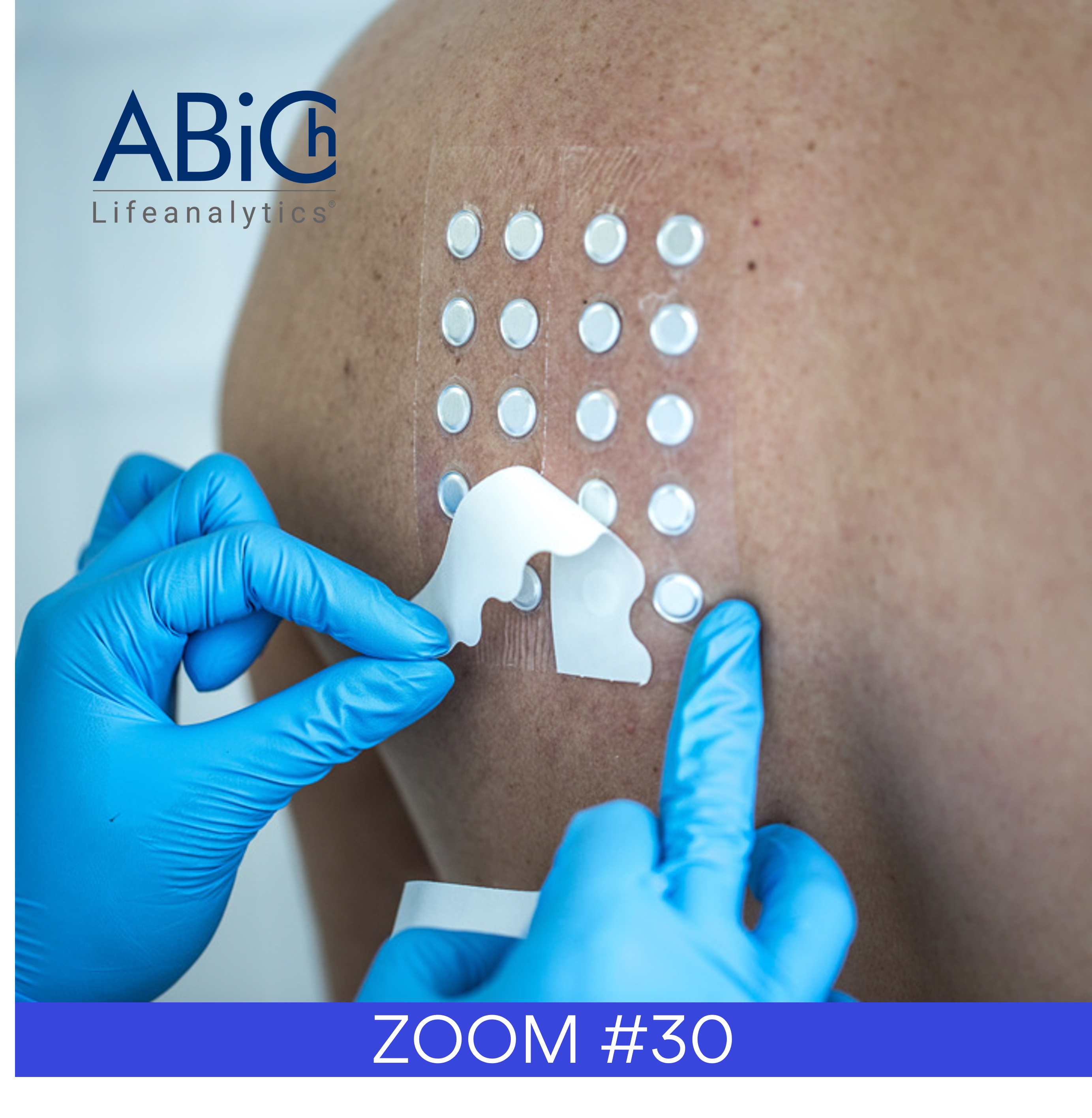
In most cases, the standard test performed to support the claim is a 48h patch test.
Contact
Elena Bocchietto
Director of the testing facility – Abich
Stefano Todeschi
CEO Abich
ABICH
Biological and chemical analysis, research and services
Verbania and Milano, Italy phone +39 0323 586239 – www.abich.it
Montreal, QB, Canada phone +1 514 507 9982 – www.abich.ca








 Follow us on Linkedin!
Follow us on Linkedin!