The determination of skin sensitisation potential is one of the most important toxicological endpoint in the development and evaluation of ingredients used in fragrance, cosmetic and personal care products.
Since the total animal testing ban in March 2013, in vitro skin sensitisation tests are one of the current hot topics in the world of cosmetic ingredients evaluation. During the last few years numerous of in vitro tests have been developed.
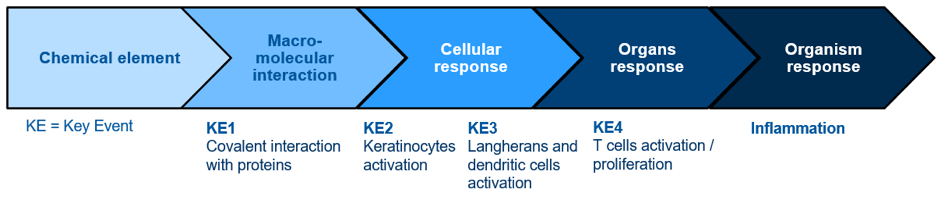
The approach used for in vitro sensitisation tests development is based on the AOP concept (Adverse Outcome Pathway) summarised below:







 Follow us on Linkedin!
Follow us on Linkedin!
You must be logged in to post a comment.